Phimostop is a certified medical device consisting of 22 medical silicone elements designed to apply the well-established technique of skin dilation to the phimotic ring and solve phimosis without circumcision.

control_point Recommended by 80% of patients.
control_point Medical device registered with the Ministry of Health
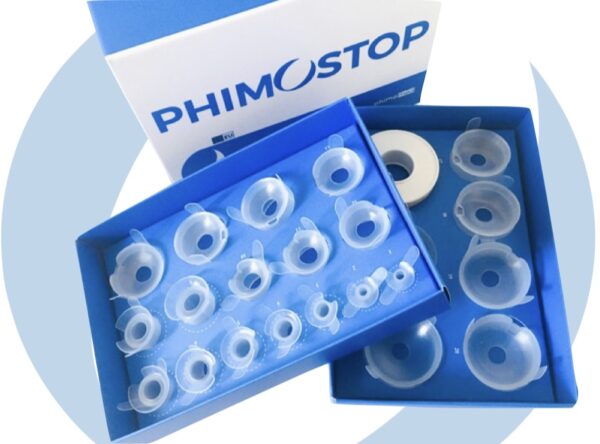
The elements are specially designed to be positioned on the glans and perform a progressive and gentle dilation directly on the part affected by shrinkage.
Through the constant application of Phimostop, the scar ring is weakened and thinned, allowing the growth of new elastic cells that replace, in a short period of time, the inelastic ones.
Patented and registered with the Italian Ministry of Health, Phimostop is suitable for everyone and provides a viable solution for all types of phimosis.
Also available with interest-free payment in instalments
Tested and effective medical device
The effectiveness of Phimostop as an alternative to circumcision surgery has also been demonstrated by data from clinical research conducted at the Tor Vergata University Polyclinic in Rome coordinated by Prof. Miano.
Phimostop is a medical device registered with the Italian Ministry of Health for the treatment of phimosis as an alternative to circumcision surgery.
The research examined the product to evaluate its efficacy as a remedy for phimosis as an alternative to circumcision surgery.
The expected results were exceeded and more than 80% of patients recovered from phimosis without recourse to surgery.
How it works
Phimostop is a medical device composed of 22 medical silicone tubes that helps treat phimosis.
By dilating the epidermal tissue, the device acts directly on the affected area,
blocking the progression of phimosis and improving the quality of sexual life.
Types of phimosis
Phimostop treats acquired phimosis by stimulating the creation of new elastic cells to replace the inelastic ones in the phimotic tissue Phimostop also treats congenital phimosis by inducing the growth of the diameter of the foreskin.
Without circumcision
Phimostop offers an alternative for those who want to avoid circumcision. A natural method that uses the principle of tissue dilation to resolve phimosis without surgery.
Phimostop customer reviews
Serious and reliable company The product is exactly as described on the website. I haven't finished the treatment yet but the results are already visible. In addition, I had lost one of the useful components for the correct use of the kit. I got in touch by email and the company responded quickly, offering to send me a new complete kit free of charge. Which they did promptly. In my opinion, this is a sign of respect and customer care.
Healed great Healed great. At the last tube I was about to give up, it wasn't very easy but worth it. My sensations during sexual intercourse are totally different, top
Finally sex without a condom! I started with a 2nd level phimosis, the foreskin was retracted by 30/40% and it was almost impossible to uncover the glans during erection. This caused me discomfort in sexual intercourse, so much so that I always had to use a condom. Surgery was out of the question, so on 14 August 2023 I decided to purchase Phimostop. I started the treatment on 16 August, starting with the 9th tuboid. I wore each tubeid for about 7 to 8 hours a day (at night) for 4 days each. After less than a month I got to the 14th tubeid. The foreskin finally retracted 100% and I was able to fully uncover the glans. On 16 September I finally had sexual intercourse without a condom and with the glans uncovered. I recommend this product! It really works!
How does Phimostop work?
To use Phimostop, simply apply the 22 silicone tubes to the part of the penis affected by phimosis. With a convenient fastening system, you can wear it during the day and night for a gradual and gentle treatment of phimosis.
Phimostop is an alternative treatment to circumcision for phimosis, consisting of 22 medical silicone tubes that use the dilation technique to treat phimosis and thus the scar ring. With a comfortable fastening system, the tubes can be worn day and night, allowing the growth of new elastic cells to replace the inelastic ones and facilitating the uncovering of the glans.
Phimostop
PHIMOSTOP solves the condition of phimosis without surgery by progressively dilating the phimotic ring and promoting the formation of new elastic cells that stabilise the result.
Safe
Validated by the Ministry of Health, EU Patent and CE Marking
For everyone
Class I medical device available without prescription
Maximum Protection
Free shipping with anonymous parcel and no reference to the product
Effective
Suitable against both acquired and congenital phimosis.
Also available with interest-free payment in instalments
"81% of patients who achieved primary endpint confirmed resolution of their phimosis 24 months after using Phimostop, thus avoiding circumcision."
Division of Urology, Fondazione PTV Policlinico Tor Vergata, Rome, Italy & Department of Surgical Sciences, Division of Urology University of Rome Tor Vergata, Rome, Italy
University of Rome Tor Vergata, Rome, Italy
Scopri di più sul nostro Blog
Read our Blog articles to find out more about Phimostop, phimosis and treatment



